
|
Caspase-3 apoptosis sensor Casper3-GR
- Early detection of Caspase-3 activity onset
- High sensitivity
- Direct expression in cells
- No exogenous chemical compounds required
- Proven suitability for FLIM-based screenings
- Recommended for early detection of apoptosis
 |
Caspase-3 (CPP32, apopain, YAMA), a member of asparate-specific cysteinyl proteases (or caspases) family, is a key mediator of apoptosis of mammalian cells [Kothakota et al., 1997]. Caspase-3 is activated during the early stages of apoptosis by self-proteolysis and/or cleavage by another protease. Active caspase-3 cleaves and activates caspases and many other cellular proteins, leading to apoptotic chromatin condensation and DNA fragmentation in all cell types examined [Porter and Janicke, 1999].
Casper3-GR is a FRET based sensor that can be used for detection of caspase-3 mediated apoptosis in living cells. The sensor consists of green and red fluorescent proteins TagGFP and TagRFP connected by the linker containing caspase-3 cleavage sequence DEVD. The high fluorescence quantum yield of TagGFP along with the high molar extinction coefficient of TagRFP and excellent overlap of donor emission and acceptor excitation spectra result in highly effective FRET between these fluorescent proteins [Shcherbo et al., 2009].
The activation of caspase-3 during apoptosis leads to cleavage of DEVD sequence and elimination of FRET that can be detected as decrease in the red emission of TagRFP and a simultaneous increase in green emission of TagGFP. Direct monitoring of the donor/acceptor emission ratio demonstrated up to 5-fold ratio changes upon cleavage by recombinant caspase 3 in vitro. The increase in donor fluorescence intensity was at least 2-fold corresponding to a FRET efficiency of at least 50%.
|
Main properties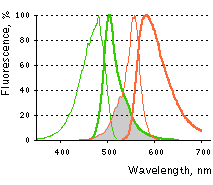
Excitation (thin lines) and emission (thick lines) spectra of TagGFP (green) and TagRFP (red) are shown individually. Spectral overlap is filled with gray.
Download Casper3-GR spectra (xls)
| | CHARACTERISTIC | |
|---|
|
| | FRET donor | TagGFP | | Fluorescence color | green | | Excitation maximum, nm | 482 | | Emission maximum, nm | 505 | | Brightness, % of EGFP | 104 | | pKa | 4.7 | | FRET acceptor | TagRFP | | Fluorescence color | red (orange) | | Excitation maximum, nm | 555 | | Emission maximum, nm | 584 | | Brightness, % of EGFP | 148 | | pKa | 3.8 | | Calculated Förster distance R0 | 5.70 | | FRET efficiency E | 0.50 | | Specificity | caspase-3 activity | | Response | elimination of FRET | | Polypeptide length, aa | 484 | | Molecular weight, kDa | 54 |
|
|---|
Recommended filter sets
The excitation wavelength required to visualize FRET changes of Casper3-GR by ratio-imaging is provided by an ordinary FITC/GFP excitation filter or ubiquitous 488 nm laser line, and the two emission signals are acquired using a 500-530 nm (FITC/GFP emission filter) bandpass filter and a 560-600 nm bandpass filter (Cy3/DsRed emission filter) or a 560LP longpass filter.
Performance and use
The excellent performance of Casper3-GR sensor has been demonstrated in vivo on staurosporine-induced apoptosis of HeLa cells [Shcherbo et al., 2009]. Living cells were monitored at 37°C with Leica SP2 confocal microscope (excitation using 488 nm laser line, emission collected at 500-530 nm and 560-650 nm). The fluorescence was evenly distributed in the cytosol and nucleus with no aggregation or non-specific localization observed. Importantly, both green and red signals were reliably stable under various irradiation conditions for hours. No reversible or irreversible fluorescence bleaching or photoconversion was observed.
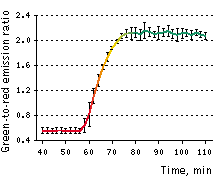
Approximately 30-40 min after 2 μM staurosporine infusion, cells demonstrated rapid (within 10 min) and pronounced changes in green-to-red fluorescence signal ratio, indicating activation of caspase-3. Later these cells demonstrated characteristic membrane blebbing. The average contrast in living cells (calculated as donor/acceptor emission ratio change for 5 cells, time point aligned to the median of ratio changes, individual for each cell) reached 3.8-fold.
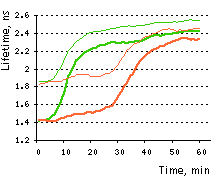
Measurement of Casper3-GR apoptosis induced FRET changes by FLIM revealed the dramatic increase of TagGFP fluorescence lifetime from 1.5 ns to 2.5 ns. The FRET efficiency of the uncleaved Casper3-GR (38% based on the phase lifetime) is among the highest measured by FLIM. Since the FRET efficiency of the cleaved substrate is zero, the dynamic range of the sensor is rather high, indicating that Casper3-GR can be successfully used for the high content FLIM based screenings on living cells.
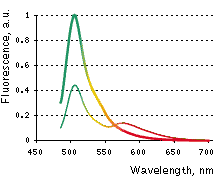 | Emission spectra of Casper3-GR before (thin line) and after digestion by Caspase-3 (thick line).
|
|---|
 |  |  | |
|---|
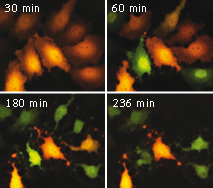
Casper3-GR upon staurosporine-induced apoptosis in HeLa cells.
Two channel fluorescence imaging of Casper3-GR upon staurosporine-induced apoptosis in HeLa cells. Time, in minutes, is shown after staurosporine infusion.
On the left graph, green-to-red emission ratio change of Casper3-GR upon staurosporine-induced apoptosis. Approximately 40-50 min after staurosporine infusion, cells demonstrated pronounced changes fluorescence signal ratio. Emission ratio shown for 5 cells, time point aligned to the median of ratio changes, individual for each cell. Excitation at 488 nm, emission was detected at 500-530 nm and 560-600 nm.
On the right graph, TagGFP fluorescence phase lifetime (thick lines) and average modulation lifetime (thin lines) changes for Casper3-GR during staurosporine-induced apoptosis. Excitation was at 488 nm and donor fluorescence emission was passed through a 500-530 nm bandpass filter.
|
Available variants and fusions
| Variant | Description | Related vector | Cat.# |
|---|
 |
|
Casper3-GR
|
When expressed in mammalian cells, this variant is evenly distributed throughout the cytosol and the nucleus.
|
pCasper3-GR
|
FP971
|
References:
-
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT.
Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis.
Science. 1997; 278 (5336):294-8. / pmid: 9323209
-
Porter AG, Janicke RU.
Emerging roles of caspase-3 in apoptosis.
Cell Death Differ. 1999; 6 (2):99-104. / pmid: 10200555
-
Shcherbo D, Souslova EA, Goedhart J, Chepurnykh TV, Gaintzeva A, Shemiakina II, Gadella TW, Lukyanov S, Chudakov DM.
Practical and reliable FRET/FLIM pair of fluorescent proteins.
BMC Biotechnol. 2009; 9 :24. doi: 10.1186/1472-6750-9-24 / pmid: 19321010
|











