
|
Kindling red fluorescent protein KFP-Red
- Reversible or irreversible photoactivation
- Activated by green light that does not damage cells and tissues
- Quenching by blue light
- Recommended for tracking cells and cellular organelle movements
 |
KFP-Red (also referred to as KFP1) is a photoactivatable GFP-like protein generated on the basis of Anemonia sulcata chromoprotein, asFP595 [Lukyanov et al., 2000; Chudakov et al., 2003a; Chudakov et al., 2003b]. KFP-Red switches from a non-fluorescent to a red fluorescent form (with excitation/emission maxima at 580 nm and 600 nm, respectively) under the exposure to intense green light irradiation. A green light laser does not damage cells and tissues. Activated KFP-Red can be easily detected because its emission spectrum is beyond the region of cell autofluorescence.
KFP-Red can be used for in vivo monitoring cell and cellular organelle movement.
|
Main properties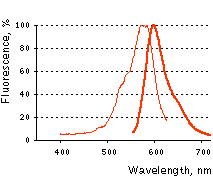
KFP-Red normalized spectra.
Download KFP-Red spectra (xls)
| | CHARACTERISTIC | before / after photoactivation |
|---|
* Brightness is a product of extinction coefficient and quantum yield, divided by 1000.
| | Fluorescence color | NO / red | | Excitation maximum, nm | 580 / 580 | | Emission maximum, nm | 600 / 600 | | Quantum yield | <0.001 / 0.07 | | Extinction coefficient, M-1cm-1 | 123 000 / 59 000 | | Brightness* | 0 / 4.1 | | Activating light | green (530-560 nm) | | Calculated contrast, fold | 35-70 | | Structure | tetramer | | Cell toxicity | not observed | | Aggregation | no | | Maturation rate at 37°C | medium | | Molecular weight, kDa | 26 | | Polypeptide length, aa | 238 | | Main advantages | Capable of reversible or irreversible kindling by green light irradiation | | Possible limitations | Limited applicability for fusions generation |
|
|---|
Recommended filter sets and laser lines
KFP-Red is non-fluorescent before light activation. Upon green-light irradiation, the protein kindles to its red fluorescent form. Green light of low intensity (e.g. 1% power scanning green laser, HeNe laser line 543 nm, 1 mW, scan per 10 seconds; the number of scans is not limited) does not cause kindling and may be used as excitation light for KFP-Red visualization.
Scanning with about 5-10% power laser results in reversible kindling of KFP-Red. More intensive-light irradiation is required for irreversible KFP-Red kindling (e.g. irradiation for 20 seconds in fast mode with a 30% power green laser light induces irreversible kindling of KFP-Red in mitochondria within the irradiated field). Irradiation with weak blue laser light causes instantaneous quenching of reversibly kindled KFP-Red, whereas for the irreversibly kindled KFP-Red, quenching is not so pronounced.
TRITC filter set or similar can be used for visualization of activated KFP-Red. Omega Optical filter sets QMAX-Red and XF174 are recommended.
Kindling effect depends on temperature. Light intensity required for kindling goes down when the temperature decreases and goes up when the temperature rises. This property can be used to achieve kindling at lower light intensities by sample cooling.
Performance and use
KFP-Red was successfully expressed and tested in various experimental models, including bacteria, Xenopus embryo, and cultured mammalian cells. Like other Anthozoa GFP-like proteins, KFP-Red is a tetramer. This restricts the wide use of KFP-Red as a fusion partner for cellular proteins.
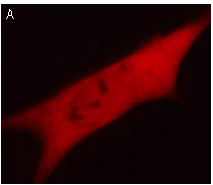 | 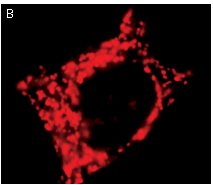 | KFP-Red use for cell (A) and mitochondria labeling (B).
Fluorescent microscopic images were obtained after reversible kindling of KFP-Red in HeLa cells.
|
|---|
Reversible or irreversible kindling: Depending on the kindling light intensity KFP-Red can be photoactivated reversibly or irreversibly allowing the monitoring of both short- and long-term cell processes.
A reversibly kindled KFP-Red relaxes to the initial non-fluorescent form (half-life 50 seconds), or can be quenched instantly by blue light (430-490 nm). Reversible kindling results in about 70 times increase of the red fluorescence intensity comparing to unkindled protein. Reversible kindling and quenching can be repeated many times.
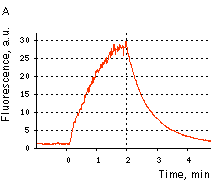 | 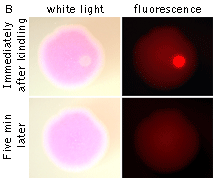 | KFP-Red reversible kindling and relaxation.
(A) Kindling and relaxation kinetics. Zero time is set at the commencement of irradiation with kindling light (532 nm laser light, 1% power). Kindling irradiation was stopped after 2 min. (B) Reversible photoactivation of KFP-Red in E. coli. The round-shaped part of the E. coli colony exp-ressing KFP-Red was irreversibly kindled with intense green light. This region fluoresces brightly, while its absorption is low. After several minutes, the kindled protein relaxed to the non-fluorescent state, while its absorption recovered.
|
|---|
An irreversibly kindled KFP-Red gives stable red fluorescence which is at least 35 times brighter than that of the protein before kindling. An irreversibly kindled KFP-Red remains stable and brightly fluorescent for more than 72 hrs in living cells and for at least a year in protein samples.
An irreversibly kindled KFP-Red can be partially quenched by blue light, but then it restores its brightness within several minutes. Therefore, in some applications, blue light can be used to quench a reversibly kindled KFP-Red, whereas an irreversibly kindled KFP-Red remains fluorescent.
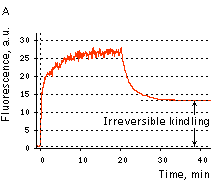 | 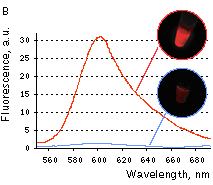 | KFP-Red irreversible kindling.
(A) Kindling kinetics. Zero time is set at the commencement of irradiation with kindling light (532 nm laser light, 20% power). Kindling irradiation was stopped after 20 min. (B) Irreversibly kindled (red line) and "unkindled" (blue line) KFP-Red fluorescence spectra and brightness ratio. The photo shows intact and irreversibly kindled KFP-Red samples after a year of incubation at room temperature.
|
|---|
Application of KFP-Red to track cell migration was demonstrated using embryonic fate mapping as an example. Xenopus embryos were taken at the stage of two blastomeres and KFP-Red mRNA was microinjected into the animal poles of both blastomeres. At the early neurula stage, a round-shaped group of cells within the neural plate was kindled irreversibly. Irradiated cells became brightly fluorescent and their migration in the developing embryo was monitored. Longitudinal extension accompanied by transversal convergence of the labeled group of cells was visible after the first two hours after kindling. At the end of neurulation, the labeled spot appeared as a thin stripe on the surface of the left neural fold.
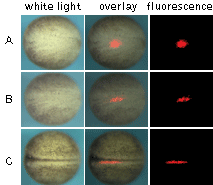 | Monitoring of cell migration during Xenopus neural plate development using KFP-Red.
(A) At the early neurula stage, a round-shaped group of cells within the neural plate was irreversibly "kindled"; (B) longitudinal extension of the labeled group of cells after two hours after kindling; (C) thin stripe of the labeled cells at the end of neurulation.
Experimental data were presented by Dr. A. Zaraisky, Institute of Bioorganic Chemistry, RAS (Moscow, Russia).
|
|---|
KFP-Red suitability for tracking movement of cell organelles was demonstrated on PC12 cells transfected with a mitochondria-targeted KFP-Red expressing vector. After 25 hours of incubation, mitochondria remained non-fluorescent (no kindling observed) upon irradiation using a 1% power scanning green laser (HeNe laser line 543 nm, 1 mW, once per 10 seconds; the number of scans is not limited). After several scans with a 5-10% power laser, mitochondria became brightly fluorescent and were observed using a 1% power laser for several minutes. Brief irradiation (about 20 seconds in fast mode) with a 30% power green laser light induced irreversible kindling of KFP-Red in mitochondria within the irradiated field. Irreversibly kindled mitochondria were monitored.
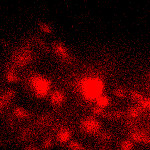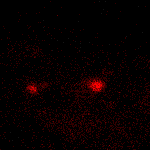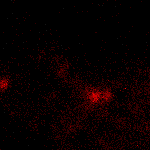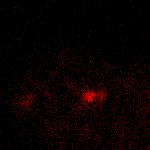 | Monitoring of mitochondria movement in PC12 cells using KFP-Red (movie).
|
|---|
KFP-Red diffusion within an eukaryotic cell was also demonstrated. HeLa cells were transfected with pKindling-Red-N vector. After 24 hours of incubation they were observed in the Zeiss LSM 510 confocal microscope. Almost no fluorescence could be observed upon irradiation by 1% power of 1mW 543 nm laser. The upper part of the cell was shortly (about 5-10 seconds) irradiated by 100% power 543 nm laser, causing kindling of the KFP-Red. Starting immediately after kindling, diffusion of the activated protein was observed using 1% power laser as an excitation light source.
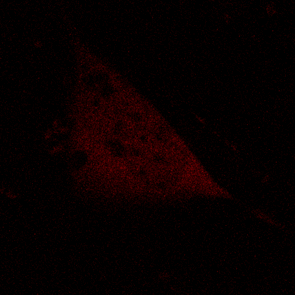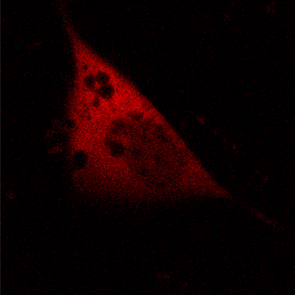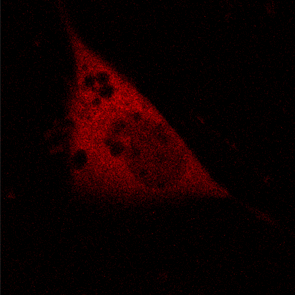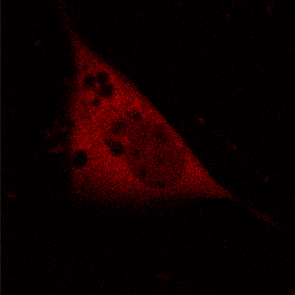 | KFP-Red diffusion within eukaryotic cell (movie).
|
|---|
See additional examples of KFP-Red use at www.olympusconfocal.com
Available variants and fusions
| Variant | Description | Related vector | Cat.# |
|---|
 |
|
Humanized KFP-Red
|
KFP-Red codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in many other heterological systems.
|
pKindling-Red-N
|
FP301
|
References:
-
Chudakov DM, Belousov VV, Zaraisky AG, Novoselov VV, Staroverov DB, Zorov DB, Lukyanov S, Lukyanov KA.
Kindling fluorescent proteins for precise in vivo photolabeling.
Nat Biotechnol. 2003a; 21 (2):191-4. / pmid: 12524551
-
Chudakov DM, Feofanov AV, Mudrik NN, Lukyanov S, Lukyanov KA.
Chromophore environment provides clue to "kindling fluorescent protein" riddle.
J Biol Chem. 2003b; 278 (9):7215-9. / pmid: 12496281
-
Haas J, Park EC, Seed B.
Codon usage limitation in the expression of HIV-1 envelope glycoprotein.
Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
-
Lukyanov KA, Fradkov AF, Gurskaya NG, Matz MV, Labas YA, Savitsky AP, Markelov ML, Zaraisky AG, Zhao X, Fang Y, Tan W, Lukyanov SA.
Natural animal coloration can Be determined by a nonfluorescent green fluorescent protein homolog.
J Biol Chem. 2000; 275 (34):25879-82. / pmid: 10852900
|
















